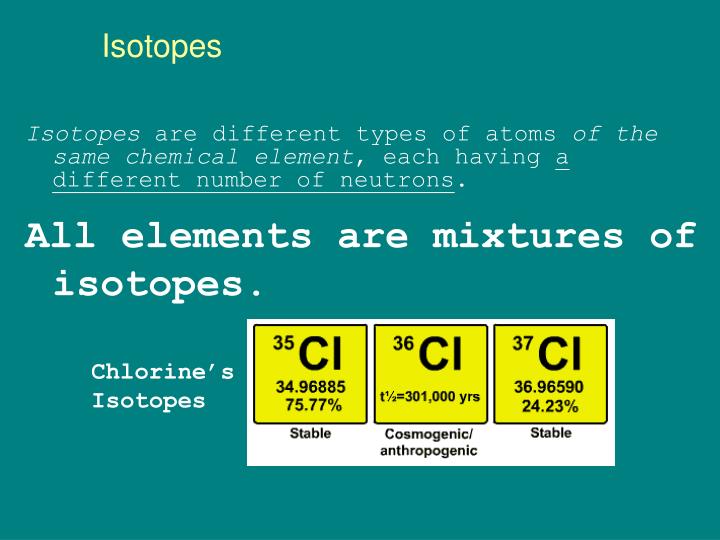
Chlorine Isotopes Notation . \( ^{35}_{17}{\rm cl}\) and \( ^{37}_{17}{\rm. isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number,. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. the symbols for the two naturally occurring isotopes of chlorine are written as follows: Given the hyphen notation, write the nuclear notation. Refer to the periodic table as needed. this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins,. to write the symbol for an isotope, place the atomic number as a subscript and the mass number (protons plus neutrons) as a.
from www.slideserve.com
to write the symbol for an isotope, place the atomic number as a subscript and the mass number (protons plus neutrons) as a. this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins,. isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number,. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. the symbols for the two naturally occurring isotopes of chlorine are written as follows: Given the hyphen notation, write the nuclear notation. \( ^{35}_{17}{\rm cl}\) and \( ^{37}_{17}{\rm. Refer to the periodic table as needed.
PPT Chapter 3 PowerPoint Presentation ID4976256
Chlorine Isotopes Notation \( ^{35}_{17}{\rm cl}\) and \( ^{37}_{17}{\rm. this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins,. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. \( ^{35}_{17}{\rm cl}\) and \( ^{37}_{17}{\rm. isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number,. Given the hyphen notation, write the nuclear notation. to write the symbol for an isotope, place the atomic number as a subscript and the mass number (protons plus neutrons) as a. the symbols for the two naturally occurring isotopes of chlorine are written as follows: Refer to the periodic table as needed.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation ID4976256 Chlorine Isotopes Notation Given the hyphen notation, write the nuclear notation. isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number,. to write the symbol for an isotope, place the atomic number as a subscript and the mass number (protons plus neutrons) as a. 53. Chlorine Isotopes Notation.
From www.savemyexams.co.uk
Isotopes (6.1.4) OCR Gateway GCSE Physics Revision Notes 2018 Save Chlorine Isotopes Notation \( ^{35}_{17}{\rm cl}\) and \( ^{37}_{17}{\rm. Given the hyphen notation, write the nuclear notation. isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number,. Refer to the periodic table as needed. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl. Chlorine Isotopes Notation.
From www.sciencephoto.com
Isotopes of chlorine, illustration Stock Image C028/6463 Science Chlorine Isotopes Notation Given the hyphen notation, write the nuclear notation. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. \( ^{35}_{17}{\rm cl}\) and \( ^{37}_{17}{\rm. isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine. Chlorine Isotopes Notation.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Isotopes Notation 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. \( ^{35}_{17}{\rm cl}\) and \( ^{37}_{17}{\rm. to write the symbol for an isotope, place the atomic number as a subscript and the mass number (protons plus neutrons) as a. the symbols for the two. Chlorine Isotopes Notation.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Chlorine Isotopes Notation to write the symbol for an isotope, place the atomic number as a subscript and the mass number (protons plus neutrons) as a. isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number,. 53 rows chlorine (17 cl) has 25 isotopes, ranging. Chlorine Isotopes Notation.
From www.expii.com
Isotope Notation — Overview & Examples Expii Chlorine Isotopes Notation the symbols for the two naturally occurring isotopes of chlorine are written as follows: \( ^{35}_{17}{\rm cl}\) and \( ^{37}_{17}{\rm. Given the hyphen notation, write the nuclear notation. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Refer to the periodic table as needed.. Chlorine Isotopes Notation.
From schematicsarjairllixq7.z22.web.core.windows.net
Chlorine Electric Dot Diagram Chlorine Isotopes Notation the symbols for the two naturally occurring isotopes of chlorine are written as follows: \( ^{35}_{17}{\rm cl}\) and \( ^{37}_{17}{\rm. to write the symbol for an isotope, place the atomic number as a subscript and the mass number (protons plus neutrons) as a. Given the hyphen notation, write the nuclear notation. this table shows information about naturally. Chlorine Isotopes Notation.
From www.britannica.com
chlorine Uses, Properties, & Facts Britannica Chlorine Isotopes Notation 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Given the hyphen notation, write the nuclear notation. to write the symbol for an isotope, place the atomic number as a subscript and the mass number (protons plus neutrons) as a. this table shows. Chlorine Isotopes Notation.
From www.alamy.com
chlorine chemical element isotopes atomic structure illustration Chlorine Isotopes Notation Given the hyphen notation, write the nuclear notation. Refer to the periodic table as needed. \( ^{35}_{17}{\rm cl}\) and \( ^{37}_{17}{\rm. to write the symbol for an isotope, place the atomic number as a subscript and the mass number (protons plus neutrons) as a. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52. Chlorine Isotopes Notation.
From present5.com
Isotopes Atoms of the same element with Chlorine Isotopes Notation 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. \( ^{35}_{17}{\rm cl}\) and \( ^{37}_{17}{\rm. the symbols for the two naturally occurring isotopes of chlorine are written as follows: isotope notation, also known as nuclear notation, is important because it allows us to. Chlorine Isotopes Notation.
From periodictable.me
How To Find The Electron Configuration For Chlorine Dynamic Periodic Chlorine Isotopes Notation \( ^{35}_{17}{\rm cl}\) and \( ^{37}_{17}{\rm. the symbols for the two naturally occurring isotopes of chlorine are written as follows: Refer to the periodic table as needed. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. this table shows information about naturally occuring. Chlorine Isotopes Notation.
From chempedia.info
Chlorine isotopes, separation Big Chemical Encyclopedia Chlorine Isotopes Notation \( ^{35}_{17}{\rm cl}\) and \( ^{37}_{17}{\rm. the symbols for the two naturally occurring isotopes of chlorine are written as follows: to write the symbol for an isotope, place the atomic number as a subscript and the mass number (protons plus neutrons) as a. Refer to the periodic table as needed. Given the hyphen notation, write the nuclear notation.. Chlorine Isotopes Notation.
From studiousguy.com
Chlorine (Cl) Properties & Uses StudiousGuy Chlorine Isotopes Notation this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins,. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. the symbols for the two naturally occurring isotopes of chlorine are written as follows: isotope notation,. Chlorine Isotopes Notation.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Isotopes Notation 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins,. to write the symbol for an isotope, place the atomic number as a subscript and the mass. Chlorine Isotopes Notation.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo Chlorine Isotopes Notation \( ^{35}_{17}{\rm cl}\) and \( ^{37}_{17}{\rm. this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins,. Given the hyphen notation, write the nuclear notation. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. the symbols for. Chlorine Isotopes Notation.
From ameblo.jp
Chlorine Electrons exarepec1973のブログ Chlorine Isotopes Notation \( ^{35}_{17}{\rm cl}\) and \( ^{37}_{17}{\rm. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. the symbols for the two naturally occurring isotopes of chlorine are written as follows: to write the symbol for an isotope, place the atomic number as a subscript. Chlorine Isotopes Notation.
From www.youtube.com
Isotopes of Chlorine YouTube Chlorine Isotopes Notation isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number,. this table shows information about naturally occuring isotopes, their atomic masses, their natural abundances, their nuclear spins,. the symbols for the two naturally occurring isotopes of chlorine are written as follows: Given. Chlorine Isotopes Notation.
From present5.com
Isotopes Atoms of the same element with Chlorine Isotopes Notation Given the hyphen notation, write the nuclear notation. Refer to the periodic table as needed. \( ^{35}_{17}{\rm cl}\) and \( ^{37}_{17}{\rm. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. isotope notation, also known as nuclear notation, is important because it allows us to. Chlorine Isotopes Notation.